Custom nanobody services
Complex architecture of monoclonal antibody restrains their clinical and diagnosis development. Consequently, many attempts have been made to minimize the size and generate smaller binding antibody fragments. Nature has already performed the protein engineering successfully with the Camelidae family and the cartilaginous fish. These antibodies found in the Camelidae family (Bactrian camel, dromedary, llama) do not have a light chain (Hamers-Casterman et al, 1993). These antibodies bind their antigen using only the variable domain of the heavy chain (VHH). They and are called sdAb (single-domain antibody), with a size of 2 x 4 nm.
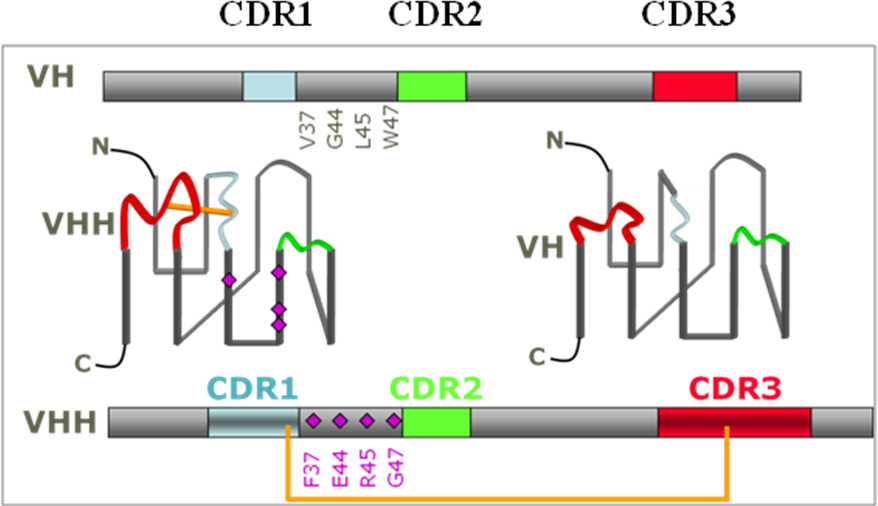

The VHH evolved in camelids in such a way that it already has the advantageous properties that scientists have tried to engineer into the isolated variable domains of classical antibodies to facilitate handling (Huang et al, 2010). These 13 kDa domains generate affinities in the low nanomolar to picomolar range, can easily be mass-produced in bacteria and are shown to be particularly resistant to different physicochemical treatments (Huang et al, 2010).
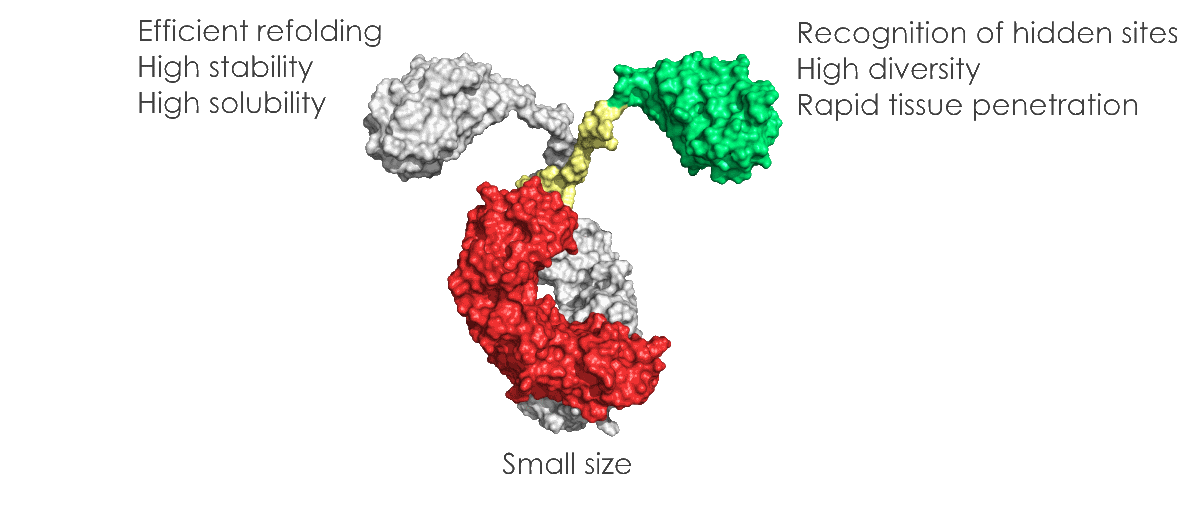
VHHs, thanks to their unique properties of size, solubility, stability, low immunogenicity, recognition of uncommon or hidden epitopes, VHHs present significant advantages compared to classical antibodies:
VHHs are naturally soluble in aqueous solution and do not have a tendency to aggregate, due to the substitution of hydrophobic by hydrophilic residues in the framework-2 region compared to conventional antibodies.
VHHs are highly heat-stable. Indeed, VHH retain more than 80% of their binding activity after 1 week of incubation at 37°C. They are also stable when exposed to the denaturing effect of chaotropic agents and in the presence of proteases or extreme pH values.
VHHs are expressed from a single gene requiring no post-translational modifications. The production process is scalable and expression systems other than bacteria can be used. There is no need of costly mammalian system for VHH expression.
VHH antibodies have many important features, such as their low inherent toxicity, that make theme valuable for biotechnological and medical applications.
The affinity of VHHs is evaluated in one digit nanomolar range in their monovalent form. In multimeric forms, affinity can frequently be reached to picomolar.
VHHs can bind into cavities or active sites of enzyme targets. Other important features are ease and speed of drug discovery and ease of manufacture.
Our services
Phase I – Immunization
You have the choice between dromedary and lama. The dromedary provides more sequence variability and better stability.
Antigens: protein, peptide, Toxins, chemical molecules, cells, cell membrane.
After seven injections, peripheral blood lymphocytes (PBLs) of the immunized dromedary or Llama were isolated and used to construct the library.
Phase II – Lymphocyte isolation, cDNA preparation, amplification and phage library
cDNA is generated via RNA isolation, amplified via PCR, and a diverse phage library is generated for screening against the antigen.
Total RNA was isolated from PBLs. To avoid contamination with VH genes, the variable regions of heavy-chain immunoglobulins (VHH) were amplified by nested PCR. The VHH was extracted from the gel. The amplified second PCR products were digested with Pst I and Not I restriction enzymes, then inserted into the phagemid. Ligation products were transformed into Escherichia coli TG1 cells by electroporation. Many clones were selected randomly and used to estimate the percentage of clones with a proper insert size within library.
A fraction of the VHH library was cultured and infected with helper phages to express the VHH at the tip of phage particles for screening against the antigen. This process represented one round of panning. The generated phage particles were used in the next round of panning. During three to four rounds of panning, Proteins-specific phages were enriched gradually.
Phase III – Antibody screening
The phage library will be screened against the desired antigen and ideally 6 potential clones will be identified and evaluated.
- In the case of unit non-response, we will re-inject the antigen (no additional charges).
Purity
Your nanobody will be delivered with a purity of >95% (SDS PAGE).
Scale
The best candidate of your nanobodies can be produced and purified in any scale. The range covers small scale production for your first experiments from 3 mg to bulk production of over 100 mg to several gram.
Storage Condition
For the delivery of your nanobody, you can choose between our nanobody storage buffer, your desired custom buffer, or even in lyophilized condition.
