Description
PhoenixDx® Cofluenza 4-Plex is a multiplex detection system for Influenza A, Influenza B, SARS-CoV-2 and a proprietary quality and performance control (HEC) based on RT-PCR. Viral RNA is converted into cDNA through reverse transcription and unique DNA sequences for each target are subsequently amplified and detected via probe-based qPCR.
Detection and discrimination between the different targets is done in 4 easy steps:
1) Collect samples: PhoenixDx® Cofluenza 4-Plex works with sputum, nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage samples in Universal Transport Medium (UTM)/ Viral Transport Medium (VTM) and swab samples.
2) Isolate RNA: sample RNA can be isolated manually with a spin column system or magnetic bead system or fully automated with an extractor like Phoenix-Pure, KingFisher™ or Tecan DreamPrep™ NAP
3) Perform RT-PCR with PhoenixDx® Cofluenza 4-Plex: PhoenixDx® Cofluenza 4-Plex allows maximum sample input of 14 µl to increase sensitivity. The HEC (human extraction control) reduces the risk of false-negative results.
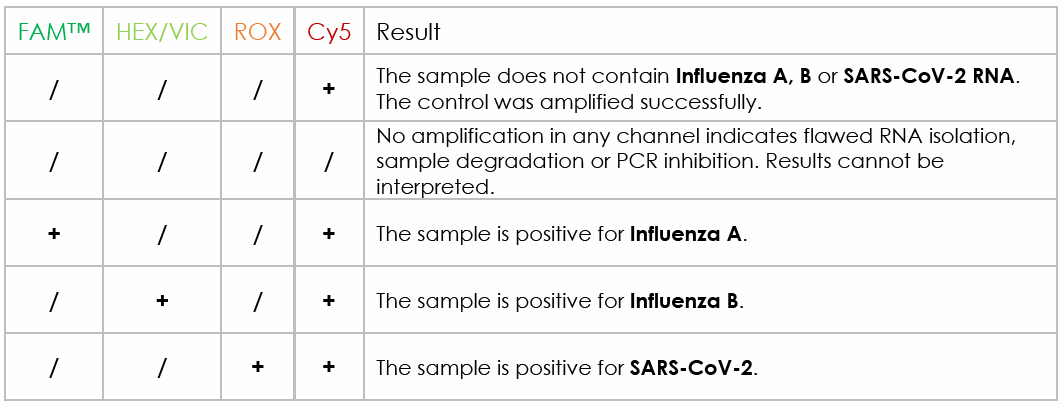
4) Analyze your results:
Download MSDS here




