Description
1) PhoenixDx® Detection System
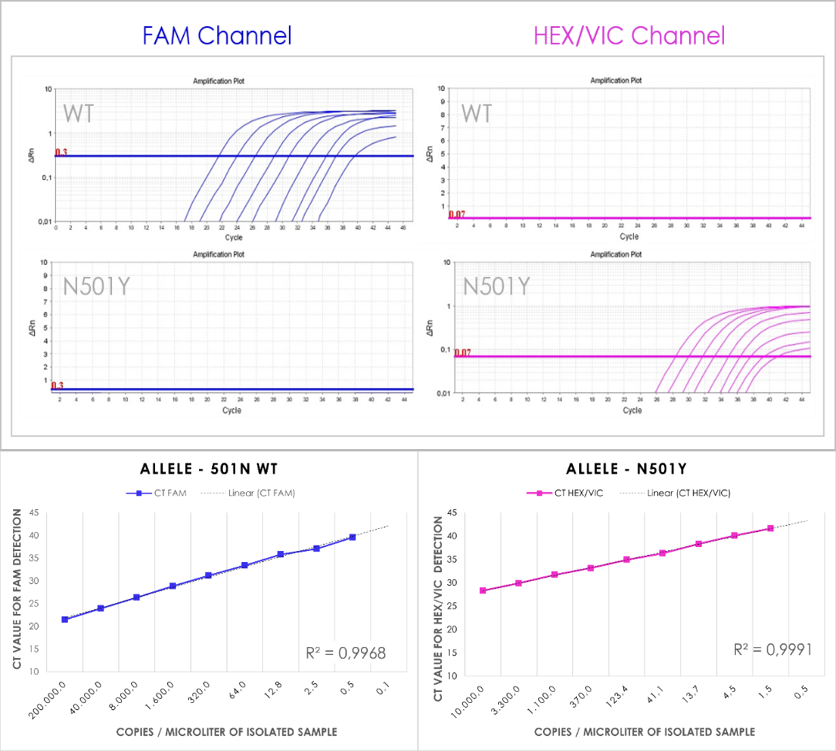
PhoenixDx® SARS- CoV-2 Mutant Screen [N501Y] is a real-time RT-PCR-based diagnostic test for the in vitro discrimination between wildtype SARS-CoV-2 and N501Y mutant SARS-CoV-2.

2) qPCR-based Detection
The first step in the discrimination between wildtype and mutant SARS-CoV-2 is the conversion of viral RNA into cDNA. Afterwards, the viral target sequences simultaneously amplified in one reaction with amplification monitored in real time using fluorescently labelled probes: upon incorporation into the newly amplified DNA strands, the fluorophore is released and an increase in fluorescence signal can be observed.
With PhoenixDx® SARS- CoV-2 Mutant Screen [N501Y], discrimination between the viral targets is achieved using of two different fluorophores that are detected in two different channels: FAM™ for the wildtype virus and HEX/VIC for the mutant virus.
Due to the intrinsic mutation rate of viruses, it is possible that mutations in the target sequence occur and accumulate over time. This can lead to false-negative results with a PCR-based detection approach.
Samples tested positive for any of the viruses should always be confirmed through complementary methods and additional analysis in an independent laboratory.
PhoenixDx® SARS- CoV-2 Mutant Screen [N501Y] is compatible with every qPCR cycler with calibrated FAM™ and HEX/VIC channel.
The kit contains 2 target positive controls (TPC, one with the wildtype target sequence and one with the mutant sequence) to verify that the PCR assays are functional.
3) Materials Provided

4) Data Interpretation

5) Exemplary Results
Download MSDS here
Download IFU here

